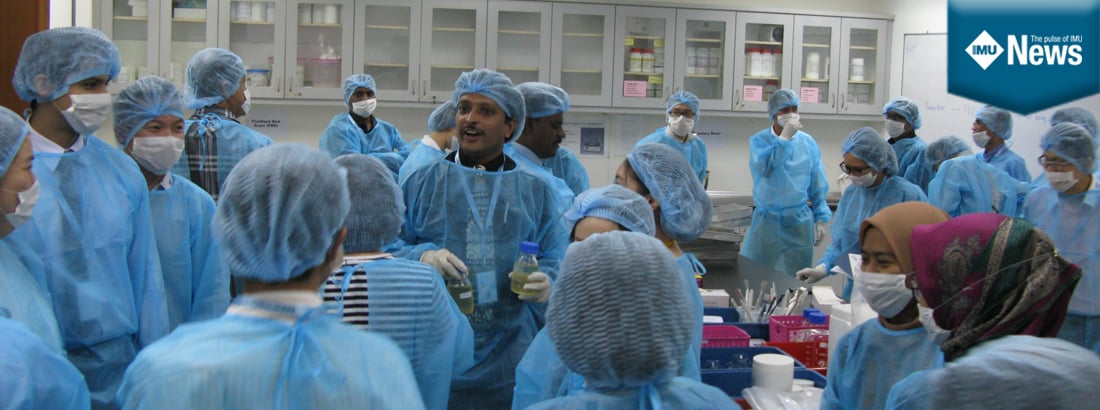
9 and 10 October 2017 – Pharmaceutical Technology Department organised a Continuous Professional Development workshop on “Granulation Technology from Bench to Market” under School of Pharmacy, International Medical University. Dr Sivaram Nallamolu and Dr Thiagarajan Madheswaran were the organising committee under the guidance of Dr Nagashekhara Molugulu along with the assistance of ICL.  This workshop, the first of its kind in Malaysia, aims to update industrial participants on advances in granulation technology through hands-on sessions and issues associated with the granulation process, and to provide networking and collaboration opportunities for researchers working in pharma industry. The new concept of industrial granulation process based on quality-by-design (QbD) were also delivered in the workshop. The workshop was a very successful event and attracted 27 participants from various Malaysian pharmaceutical industries and 8 participants from academia and one from Natural Remedies, India. During the welcome address, A/Prof Mohd Zulkefeli bin Mat Jusoh welcomed speakers and delegates and briefed the audience on the theme of the workshop.
This workshop, the first of its kind in Malaysia, aims to update industrial participants on advances in granulation technology through hands-on sessions and issues associated with the granulation process, and to provide networking and collaboration opportunities for researchers working in pharma industry. The new concept of industrial granulation process based on quality-by-design (QbD) were also delivered in the workshop. The workshop was a very successful event and attracted 27 participants from various Malaysian pharmaceutical industries and 8 participants from academia and one from Natural Remedies, India. During the welcome address, A/Prof Mohd Zulkefeli bin Mat Jusoh welcomed speakers and delegates and briefed the audience on the theme of the workshop.
The workshop was designed with a combination of plenary talks and hands-on sessions in granulation technology and covered topics that are essential in the pharma manufacturing such as high shear granulation, scale up, reproducibility issues, generic product development, granule characterization methods, risk assessment and mitigation plans.
The new concepts of product development and manufacturing by QbD was delivered by Dr Murali Mohan Babu an industrialist from Dexa Laboratories Ltd, Jakarta, Indonesia. There was also a talk by Dr Nagashekhara on problems and remedies associated in marketing of solid dosage forms that focused on dosage form design based on customer expectations. The hands-on and kahoot sessions on these topics attracted the attention of all the participants. 


The participants who attended the workshop were entitled for 10 CPD points under Category A4 which were approved by the Malaysian Pharmaceutical Society, Malaysia. Highly positive feedback were received from the participants of which they were benefited with the plenary lecture, hands-on and kahoot sessions. Most of the participants reflected that they received practical solutions for trouble shooting their problems in granulation technology and learnt basic technical skills which can be used in their regular work place.